The following sorption isotherms for wheat (Fig. 8) are intended to illustrate the types of question about the behavior of hygroscopic goods which can be answered using sorption isotherms. The sorption isotherm for 20°C indicates equilibrium states between the water content of the wheat and relative humidity.
- Higher water contents of the wheat are associated with higher relative humidities than equilibrium moisture contents and vice versa.
For example:
| Water content of wheat |
Relative humidity |
12% |
55% |
13% |
65% |
15% |
75% |
- At a measured water content of 18% (point 1) and a relative humidity of the ambient air of 75%, the point of intersection lies above the sorption isotherm. In an unventilated bulk container, the relative humidity will increase until a state of equilibrium is reached at a relative humidity of approx. 88% (point 2). The mold growth threshold, which is at 75% relative humidity, is thus exceeded. The onset of mold formation may accordingly occur in the closed container. The product's water content does not change owing to the slight release of water vapor which is required for this to happen. This situation would have to be rectified by ventilation, the cargo theoretically being dried with suitable ventilation air until its water content was just below 15% and a state of equilibrium with the ambient air had therefore been reached.
For example, if it were desired to adjust 100 metric tons of cereal with a water content of 16% to a water content of 13%, 3 metric tons of water vapor would have to be removed by ventilation; however, in practice, thorough drying of excessively moist cargo is not possible.
The grain would spoil due to mold growth in parts of the container or within the cargo (dead air zones).
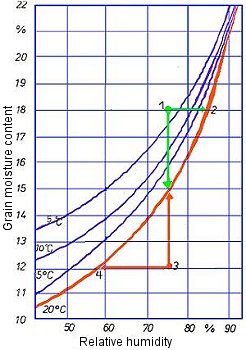 |
Figure 8: Sorption isotherms for wheat at various temperatures
[27]
|
- The water content of the product is below the sorption isotherm: (Fig. 8). At a measured water content of 12% and a relative humidity of the ambient air of 75%, the point of intersection lies below the sorption isotherm (point 3).
In an unventilated bulk container, relative humidity would decrease as a result of water vapor adsorption by the hygroscopic product until a state of equilibrium was reached at a relative humidity of just below 60% (point 4). Since the amount of water vapor to be adsorbed is small, the water content of the product hardly changes during this process. There is generally no risk of impairment of quality.
If it were possible to ventilate the bulk container constantly with air of a humidity of 75%, the grain would gain water content until the equilibrium state was achieved at 15%. In this case too, no spoilage would occur as a cereal moisture content of 15% is still within the transportable range.
Critical water content means a water content which, if exceeded during container transport or storage, must be expected to cause the onset of quality degradation, such as mold, fermentation, rot, self-heating/spontaneous combustion.
Such damage occurs at relative humidities of > 75% in the container; relative humidities of 60 - < 75% are safe storage conditions for the majority of products.
Container dry describes a product which has a water content such that it does not suffer any impairment of quality under normal weather or container conditions.
For safe transport, it is therefore important for the water content of a product to match the required values when the container is packed and for this water content to be maintained in transit through the storage climate conditions in the container.
The following factors must accordingly be taken into account in container transport:
- check water content by measurement before packing the container
- find out the intended route (climatic zones)
- determine the season of transport?
- does the water content correspond to the anticipated conditions?
To summarize:
In an unventilated container packed with a hygroscopic product, the product determines the relative humidity in the container, i.e. the product creates its own atmospheric environment. During these balancing processes, the product itself undergoes only slight changes to its water content, since this quantity of water is a multiple of the absolute humidity of the container air.
In a container which is ventilated or accessible to the air and is packed with a hygroscopic product, the relative humidity of the container air depends on the external air values, the product being able to absorb or release water vapor accordingly. It should be noted that only when a product is subjected to constant ventilation with excessively moist or dry air for several days or weeks does its water content increase or decrease slowly. It should also be noted that, even when a container is ventilated, the air between particles (e.g. cereal grains, coffee or cocoa beans) or in dead air zones behaves as if the container were closed, i.e. unventilated.
- Influence of product temperature on equilibrium moisture content:
If the temperature of the product changes, this change modifies the equilibrium humidity conditions. Since a 10°C sorption isotherm is, for example, higher than a 20°C sorption isotherm, identical water contents correspond to lower equilibrium moisture contents of the air. For example, the equilibrium moisture content for wheat with a water content of 15% and an intrinsic temperature of 20°C is approx. 75%; by contrast, at an intrinsic temperature of 10°C, the equilibrium moisture content is approx. 68%. Consequently, for example on a route to Canada or the USA, the admissible water content for cereals may be higher at the prevailing low air temperatures without the critical relative humidity of > 75% (mold growth threshold) being exceeded in transit.
For goods of vegetable origin loaded in tropical ports, these equilibrium relationships mean that it is essential to take full advantage of every opportunity to cool the goods. Warm products, such as cocoa beans from Lagos or coffee beans from Santos, release large amounts of water vapor and lead more quickly to sweat formation.
|
